|
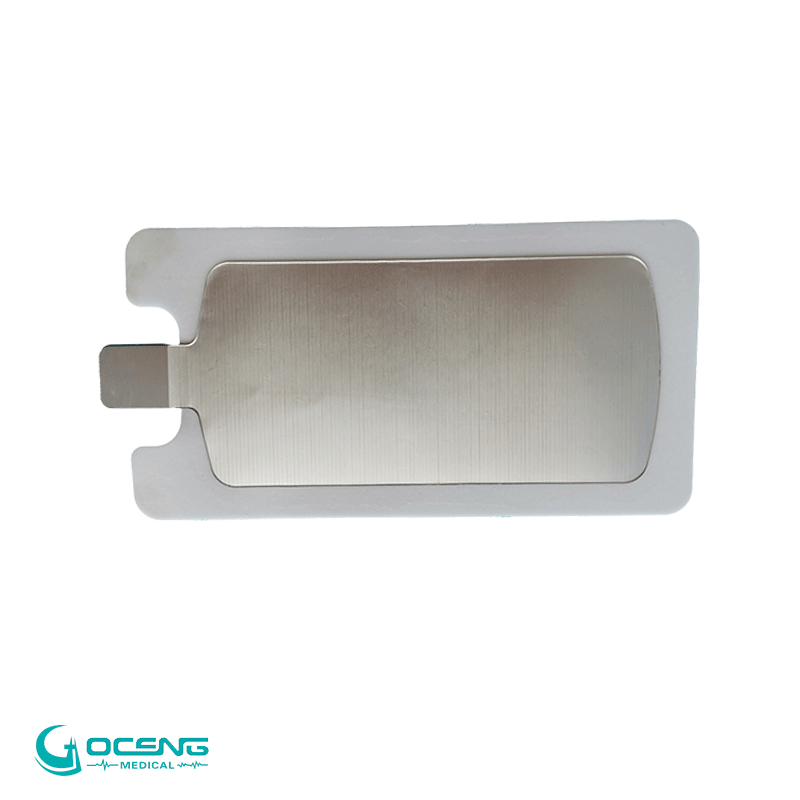
Model |
GCTE-0031 |
|
Sizes |
140*26mm ,100*26mm,100*30mm |
|
Material |
ABS ,Stainless steel |
|
plug |
2.0mm, 2.5mm,option |
|
Color |
White |
|
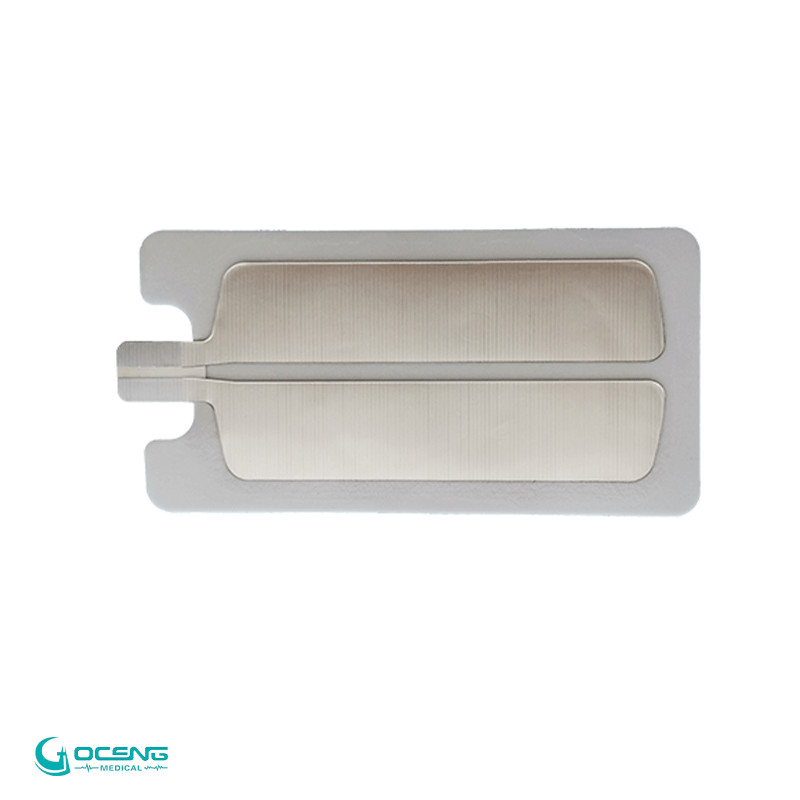
Model |
GCTE-0031 |
|
Sizes |
140*26mm ,100*26mm,100*30mm |
|
Material |
ABS ,Stainless steel |
|
plug |
2.0mm, 2.5mm,option |
|
Color |
White |
|
Packing |
EO -sterile bag , PE bag |
|
Certificate |
ISO13485 |
Contraindications